EmerAX/TracLine (intermediate catheter) & FocuStar (balloon catheter) assist in the treatment of Type III Arch RCA-C4, C6 multiple aneurysms with associated aneurysmal arterial stenosis
Surgeon team - Interventional Vascular Surgery Department, Cerebrovascular Disease Group, Peking University Third Hospital




Surgeon's profile

Dr Han Jintao
Peking University Third Hospital
- Doctor of Medicine, Chief Physician
- Deputy Director of the Interventional Vascular Surgery Department and Head of the Cerebrovascular Disease Group at Peking University Third Hospital
- Youth Committee Member of the Interventional Radiology Group, Chinese Society of Radiology
- Member of the Interventional Medicine Division and the Combined Surgery Group, Beijing Medical Association
- Member of the Neurointervention Group, Neurosurgery Division, Beijing Medical Association
- Member of the Carotid Artery Group, Vascular Surgery Physician Branch, Chinese Medical Doctor Association
- Member of the Interventional Neurosurgery Subcommittee, Chinese Stroke Society
- Vice Chair of the Neurointervention Committee, Beijing Chronic Disease Prevention and Health Education Research Association
Case information
Age: 68 years old
Gender: Male
Chief Complaint: Numbness in the left lower limb for over two years.
Present Illness: The patient developed intermittent numbness in the left lower limb over two years ago, each episode lasting 3 to 5 minutes, with relief after resting. The episodes were not accompanied by dizziness, headache, nausea, vomiting, or limb weakness. A CTA of the head and neck at an outside hospital revealed severe stenosis in the right internal carotid artery (ICA) at the C1 segment, moderate stenosis at the C4 segment, and aneurysms at the C4 and C6 segments of the right ICA. Seven months ago, the patient underwent balloon angioplasty and stent placement at the C1 segment of the right ICA at another hospital, resulting in partial symptom relief. The patient now seeks further treatment for an intracranial aneurysm and was admitted to our hospital.
Past Medical History:
– Cholecystectomy over 20 years ago.
– Balloon angioplasty and stent placement in the C1 segment of the right ICA 7 months ago.
– A history of hyperlipidemia for over 3 years.
Physical Examination:
– The patient is conscious and alert, with fluent speech.
– Bilateral pupils are equal, round, and 2.5 mm in diameter, reactive to light.
– Symmetrical facial creases, tongue protruding in the middle.
– Limb strength is graded 5 (normal) bilaterally.
Diagnosis:
1. Intracranial aneurysm (right ICA C4 and C6 segments, multiple).
2. Moderate stenosis at the right ICA C4 segment.
3. Cerebrovascular disease.
4. Hyperlipidemia.
Preoperative imaging and diagnosis



Preoperative analysis
1. Surgical Objective: Embolization of multiple aneurysms in the right internal carotid artery (ICA) at the C4 and C6 segments.
2. Key Surgical Steps: Using a long sheath, place the intermediate catheter into the proximal right ICA C4 segment. First, perform balloon angioplasty to dilate the stenosis at the right ICA C4 segment. Then, deploy a stent to cover the aneurysm neck at the C4-C6 segments of the ICA.
3. Surgical Challenges: The surgical access route is tortuous, making the placement of the intermediate catheter challenging. The patient presents with multiple aneurysms and concomitant stenosis of the proximal artery feeding the aneurysms, which carries a risk of stent deployment failure. The artery shows significant atherosclerosis, increasing the risk of poor stent apposition.
Surgical process


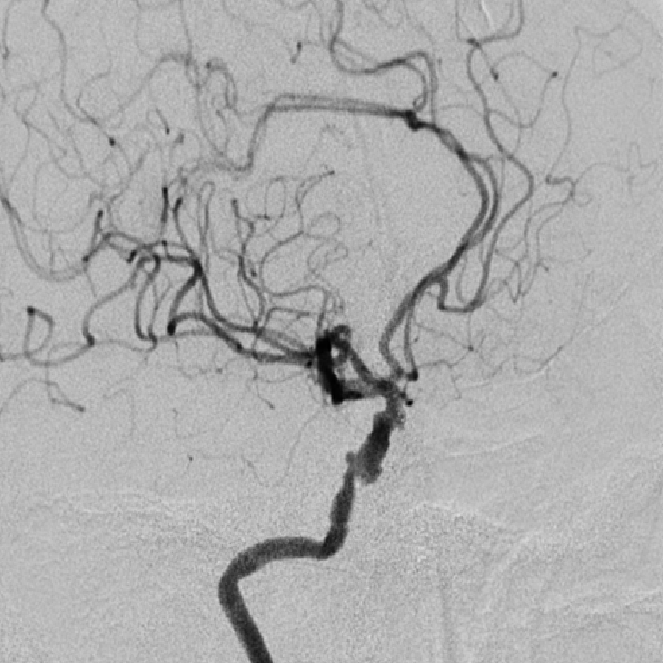
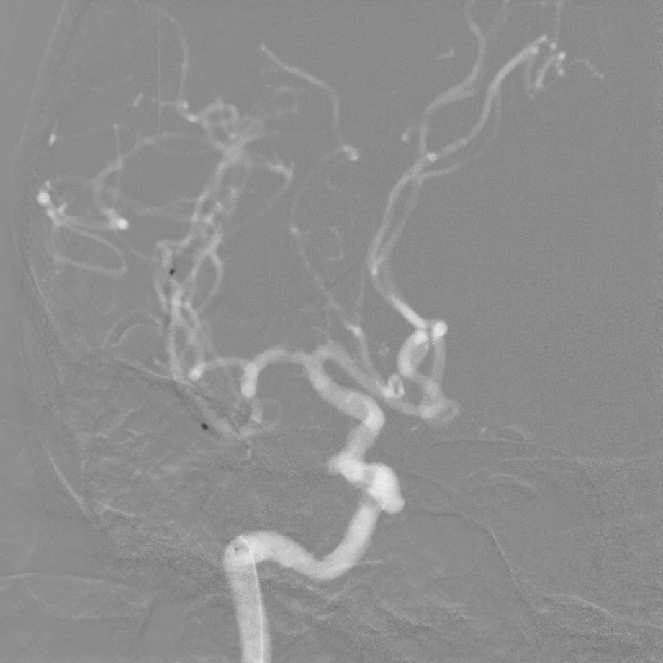
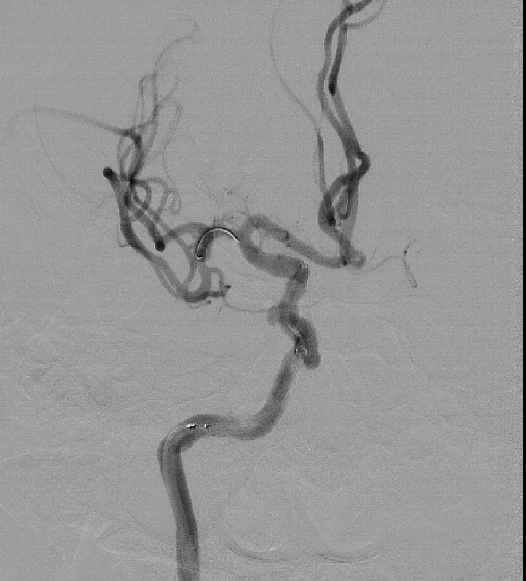
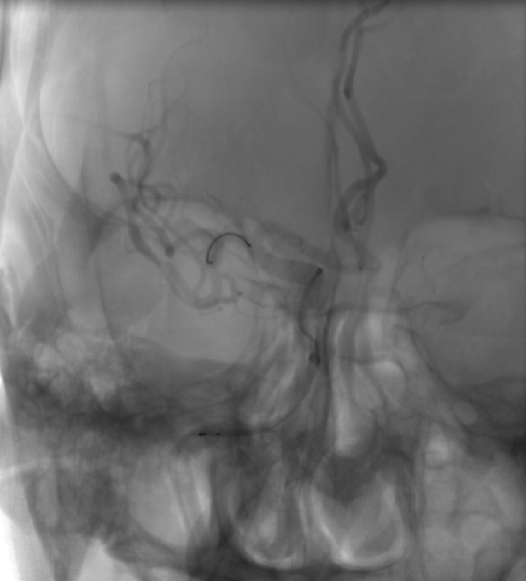

Post Operative Images


Case Summary
Case Characteristics:
This patient has a Type III aortic arch with a tortuous access route, multiple aneurysms at the C4-C6 segments requiring the placement of a stent with a dense mesh design. The intermediate catheter must have excellent trackability, be able to reach the lesion site as closely as possible, and provide stable support, as the stent requires tension release. Additionally, the patient has proximal stenosis and severe atherosclerosis in the artery feeding the aneurysms, necessitating precise and safe balloon catheter dilation.
Device Insights:
1. TracLine 071-115 catheter, with its gradually softening tip, ensures excellent passability, making it easy to reach the C4 segment. The stable support provided by the middle and proximal segments of the catheter also guarantees proper tension release during stent deployment.
2. FocuStar 3.0-15mm neuroballoon is a low-pressure (working pressure 4 atm) rapid-exchange balloon, which not only reduces the pressure gradient and shortens the dilation time but also ensures effective dilation of the stenosis while minimizing the risk of dissection. This balloon is particularly well-suited for tortuous, narrow, and fragile cerebral vessels, making it a true neuro-specific balloon.
The Product



About Hemo
Hemo Biotechnology Co., Ltd. (referred to as “Hemo”) officially commenced operations in 2017. Hemo’s global headquarters is in Singapore, with research and development centers in both Singapore and China. It collaborates with long-term partners in the United States to focus on new product design and keep abreast of cutting-edge global technologies.
Hemo is dedicated to integrating high-quality global research and development, production, clinical, and academic resources to provide innovative vascular and neurointerventional products for patients and medical professionals. It aims to offer comprehensive intervention solutions for cerebrovascular diseases, including ischemic stroke, hemorrhagic stroke, and intracranial vascular stenosis.

